Automated
Precise
Transformative
See how AmCad is Pioneering Ultrasound AIAutomated
Precise
Transformative
See how AmCad is Pioneering Ultrasound AIAmCad BioMed Corporation (AmCad) was founded in 2008 and was listed on Taipei Exchange in the March of 2015 (Ticker: 4188). Our breadth of expertise, from R&D to commercialization, have resulted in world’s first FDA cleared ultrasound Computer-Assisted Detection (CAD) devices. AmCad is committed to assisting medical professionals in making efficient and accurate diagnosis through the support of detailed sonographic visualization and quantification. By integrating AI technology with clinicians' expertise, AmCad will continue to pioneer best-in-class imaging solutions that anticipate mainstream medical needs.
We believe in a future where AI empowers physicians to make rapid and precise diagnoses, thus enabling more efficient use of medical resources. We continue to pour R&D and clinical validation resources into pioneering ultrasound AI to conquer unmet medical needs in the fields of thyroid cancer detection, Obstructive Sleep Apnea detection, real time analysis for fatty liver and liver fibrosis, and ultrasound guided intervention for MSK therapy. With the right combination of medical expertise and AI-ingenuity, we are pioneering better healthcare for all.
- Automated ultrasound scanning and detection of pharyngeal airway
- Risk evaluation of obstructive sleep apnea (OSA)
- FDA 510(k) cleared, CE certified
- FDA 510(k) cleared, CE certified
- Differentiation between pulsatile signals and noise
- FDA 510(k) cleared, CE certified
- Backscattered signals to assist with tissue variation assessment
- FDA 510(k) cleared, CE, TFDA, CFDA certified
- Assessment of malignancy potential for thyroid nodule sonographic features
- Risk analysis of malignancy for thyroid nodules
- Cytology microscopic image processing
- Visualization and quantification of clinical cytology features
- Point of care ultrasound device
- Real time AI imaging


Introducing the WORLD’S FIRST ultrasound based detection for Obstructive Sleep Apnea.
AmCAD-UO is an innovative solution to help physicians evaluate the risk of Obstructive Sleep Apnea (OSA). The evaluation process is performed on awake patients in 10 minutes using a compact ultrasound device equipped with a laser-guided positioning system and computer-assisted detection software. The automated scanning procedure not only provides the upper airway assessment but also creates 3D reconstruction images for doctors’ companion diagnostics of OSA treatment.


Introducing the WORLD’S FIRST ultrasound CAD for Thyroid Cancer Detection.
AmCAD-UT® Detection gives physicians a tool to process ultrasound images for sonographic characteristics that assist in making diagnostic decisions.
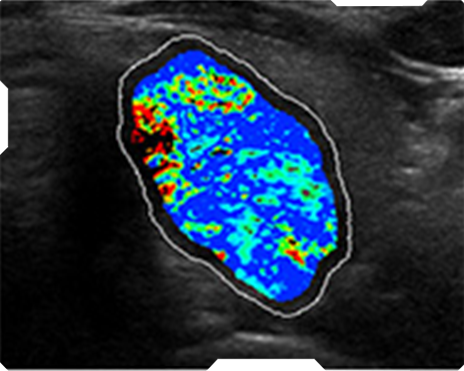
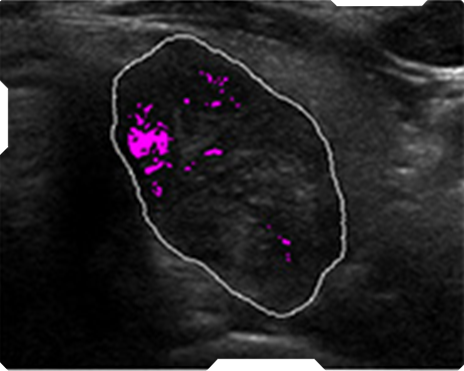
AmCAD-UT® Detection uses statistical pattern recognition and quantification methods to perform analytical processing of images. By processing the image for key characteristics (i.e., echogenic foci, echogenicity, texture, margin, anechoic areas, height/width ratio, nodule shape, and nodule size). AmCAD-UT® Detection provides doctors with quantification and visualization of the sonographic characteristics required for better informed decision making.
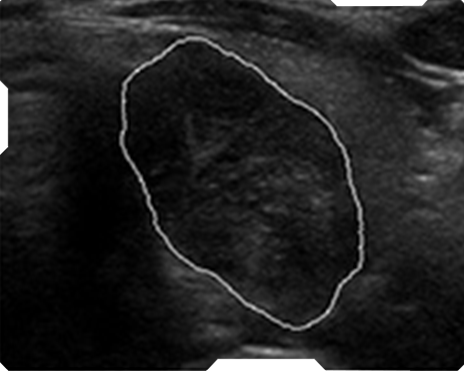
Automate Nodule
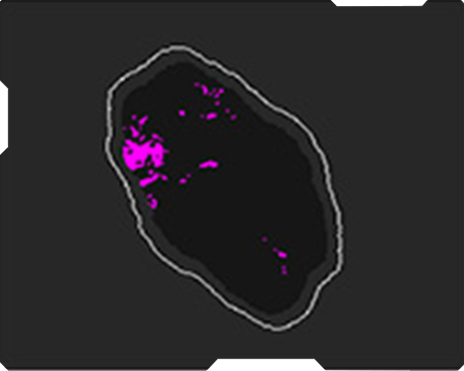
Echogenicity
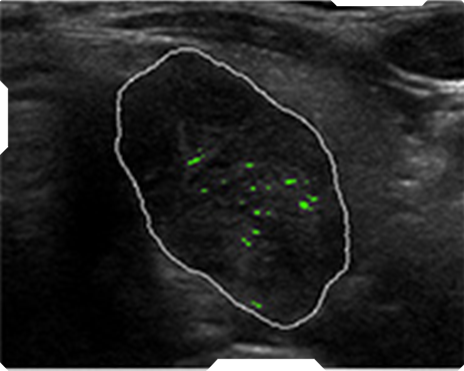
Echogenic foci
Texture
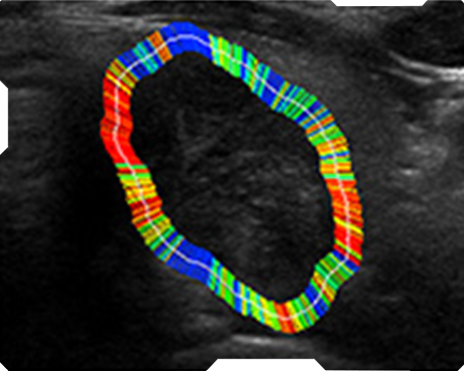
Margin
Automated Nodule Recognition

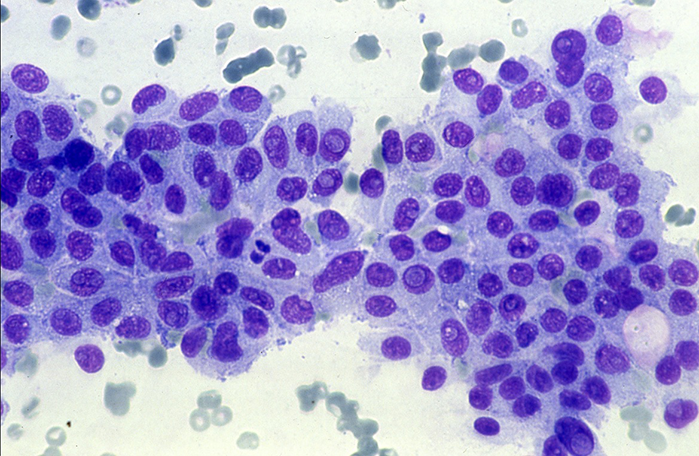
AmCAD-CA, specially designed for microscopy cytology, is the only commercially available software specifically designed for cytopathology analysis.
《Commercial Times》AmCad and Swiss Hospital Reveal Promising New Tech for OSA Treatment Success
+ MORE《Economic Daily News》AmCad & Stanford Sleep Center's research results published in "Sleep Medicine"
+ MOREISSS Annual Meeting 2024
+ MORE